It’s been recognized in beer since the 1870’s, and it may just be one of the most well-known flavor defects in beer across the world. Just because it’s recognizable, however, doesn’t mean people are necessarily bothered by it since Corona and Heieneken sell plenty of bottled beer. It’s becoming common knowledge among some of the beer-drinking public that putting beer in clear or green bottles will allow it to become skunky or “lightstruck”. Following right behind is the growing awareness that, with the use of specialized hop extracts, brewers can successfully put beer in such bottles without resulting in skunked beer (Miller products being the best known of these). We’ll discuss these topics and more as we explore this phenomenon. A warning: there will be chemistry.
First, the molecule: 3-methylbut-2-ene-1-thiol, but you can call it 3-MBT for short. With a threshold of around 4 parts-per-trillion in beer, 3-MBT is among the most potent flavor compounds that can be found in beer; as such, it does not take much to ruin your beer. If you drink your beer from a pint glass on a sunny patio you may notice this flavor by the time you reach the bottom of the glass – that’s how quick this problem can arise.
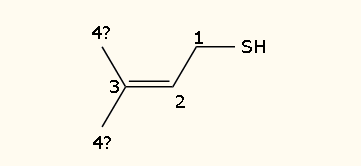
Here’s the little bugger now. Rather innocuous looking, isn’t he?
We’ll take a small break here for a minor organic chemistry lesson involving molecular nomenclature. The great thing about O-chem is that there are rules by which molecules are named, and if you know the rules you can figure out the molecule’s shape and features. Let’s take 3-methylbut-2-ene-1-thiol: the “but” part of the name tells you that there we are dealing with a carbon-chain that is 4 carbons long (1 carbon: meth-; 2 carbons: eth-; 3 carbons: prop-; 4: but-; 5: pent-; etc etc). Each carbon is numbered sequentially, and I’ve included the numbers in the image. As the name implies, there is a “thiol” group on the number 1 carbon. A thiol is like an alcohol group but with a sulfur atom in place of an oxygen, -SH instead of -OH. Continuing to look at the name we see that on the number 2 carbon there’s an “ene” group, which means that there is a double bond between that carbon and the next. Finally, on the number 3 carbon, there is a methyl group (essentially a branch made up of a carbon “chain” made of only one carbon – remember “meth-” meaning 1 carbon?). So, if one of the carbons off of #3 is a methyl group, which one is the #4 carbon? For the purposes of this molecule, it can be either one, and whichever is #4 then the other is the methyl group. This concludes the nomenclature lesson. Now, back to the… well, more chemistry I guess.