One of the most ubuiquitous flavors in beer, present to some degree in pretty much every beer, is dimethyl sulfide, or DMS. It’s a normal part of beer flavor but, as usual, its acceptability is dependent on the intentions and desires of the brewer. It can be a large portion of the flavor profile of certain beers, while in other beers it is expected to be at much lower levels. For example, Rolling Rock is considered to be a prominent example of a beer which is high in DMS (although in the past it may have been swamped by skunky/lightstruck flavors as I believe Rolling Rock has not always been brewed with light-stable hop extracts).
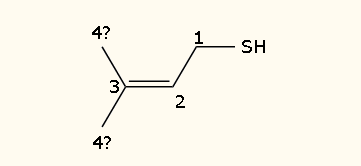
DMS has the aroma of canned vegetables, particularly corn or creamed corn. It’s a small and simple molecule; as the name conveniently implies, it has two methyl-groups flanking a sulfur atom:

Typical flavor threshold for DMS in beer is about 35ppb, and beers from around the world can contain anywhere from 10-200ppb. Typically lagers tend to have a bit more DMS than ales do, but what dictates the DMS levels in your beer more than the yeast is the production parameters in your brewery, particularly the kettle boil and wort-chilling.
DMS is considered to originate from malt, although it is actually formed in the brew kettle. All malt contains a variant of the amino acid methionine called S-methyl methionine (SMM), and it is an intermediate in a number of biosynthetic pathways which plants use to make other compounds. SMM is pulled into the wort during mashing and lautering and as the wort is heated the SMM degrades and is converted into DMS. While the wort is boiled, however, the volatility of DMS allows it to be removed from the wort and out the ventilation stack to never return. If there is no way for steam condensate to escape from your kettle (like if your homebrew pot is covered during the boil) then that condensate will drip back into the wort returning that DMS back into your beer. In such a case, the SMM is still creating DMS because the wort is hot, but the DMS can’t go anywhere so it just continues to build up into higher and higher levels. For this reason, the whirlpool and/or wort-chilling stage after the boil is a critical time in DMS control: if the wort sits too hot for too long, DMS will continue to develop since there is no boil to drive off the compound. I recall some power outages here at the brewery which knocked out the brewhouse for a bit. The brew that was in the whirlpool at the time wound up staying in there for much longer than it should have, which lead to a beer with astronomical DMS levels. At first glance, you may think that controlling DMS might be as easy as making your boils longer to convert all the SMM to DMS and drive it out the stack, but there is enough SMM in most malts that this is not a practical solution: the boil times required to convert it all and volatilize it could be hours long and would be quite detrimental to other wort parameters and the quality of the beer. Some kettles are more efficient at stripping DMS than others, as well. A simple direct fire or steam-jacketed kettle would be less efficient at removing DMS than a kettle with a calandria, which would be less efficient than a Merlin kettle. In fact, I recall brewery which, after installing a Merlin-style kettle, wound up being so efficient at stripping volatiles from the wort that they had to “de-tune” the kettle to make it less efficient since it was throwing off the flavor profile of their beers.
Even after the wort is chilled to the point where SMM is no longer being converted into DMS, the whole corny story isn’t over yet. During fermentation, the carbon dioxide that is produced by the yeast has a scrubbing effect on the DMS, carrying some of it out of the beer. This happens more efficiently at higher temperatures since the fermentations are more vigorous, and for this reason ale fermentations are better at this than lagers. This is why many lagers tend to have somewhat higher levels of DMS than ales do.
Here is a pretty handy chart I found on the internets showing some data about the DMS levels in wort/beer over the course of production. You can see how the levels drop significantly during boiling, but how they can potentially rise again before the wort is chilled. Then they fall again during fermentation as the yeast help blow off some more of the remaining DMS.

Finally, another potential source of DMS can actually come from bacterial infection. Some species of Enterobacter can produce DMS, along with diacetyl. This is quite uncommon in normal production scenarios, but it could conceivably happen more frequently in homebrewing situations. However, the vast majority of DMS in beer comes from the malt and the boil, so if you have an issue with corny beer, check the brewhouse parameters first.
But the story of corny beer doesn’t stop there, no! A challenger appears!
As I collected various flavor compounds that I understood were in beer, I came across a reference to another malt-based compound which was described as “biscuity/malty”. I thought this might have something to do with the biscuity aroma which is a dominant malt flavor in beers which use Victory or “biscuit malt”. Well, I was wrong. As I opened the package of 2-acetylpyridine which Sigma-Aldrich had shipped to me, I realized “This isn’t biscuity at all. This smells like freshly cooked corn tortillas!” It was like opening up that container of steaming tortillas at a Mexican restaurant, or a bag of high quality corn chips. So that’s what my panel calls 2-acetylpyridine now, “corn chips” rather than “biscuity”. Wikipedia says that 2-ap has an odor threshold of about 60 parts-per-trillion, but other literature values I’ve seen indicate that in beer it is closer to 40 ppb (and my experience with it shows this to be pretty close).
2-acetylpyridine, as seen below, is found in malt (and corn chips) and is created by the Maillard browning reactions. These reactions take place when certain types of sugars are heated in the presence of amino acids. It’s a highly complex series of reactions that take place which lead to a whole slew of compounds, including flavor compounds and color compounds. It’s not caramelization, but it can be confused with it if you are unfamiliar with the differences. The browning of the bread as it toasts, the malting of barley, the browning of beef as it cooks – these are examples of Maillard browning reactions.
I’m not going to go much into 2-ap, but just brought it up to show that not all corn-type flavors in beer come from DMS. In fact, I’m starting to think that some of the flavors in our beer that I have previously associated with low levels of DMS might actually wind up being 2-ap and that’s pretty interesting.

Hope to post again soon! But probably not.